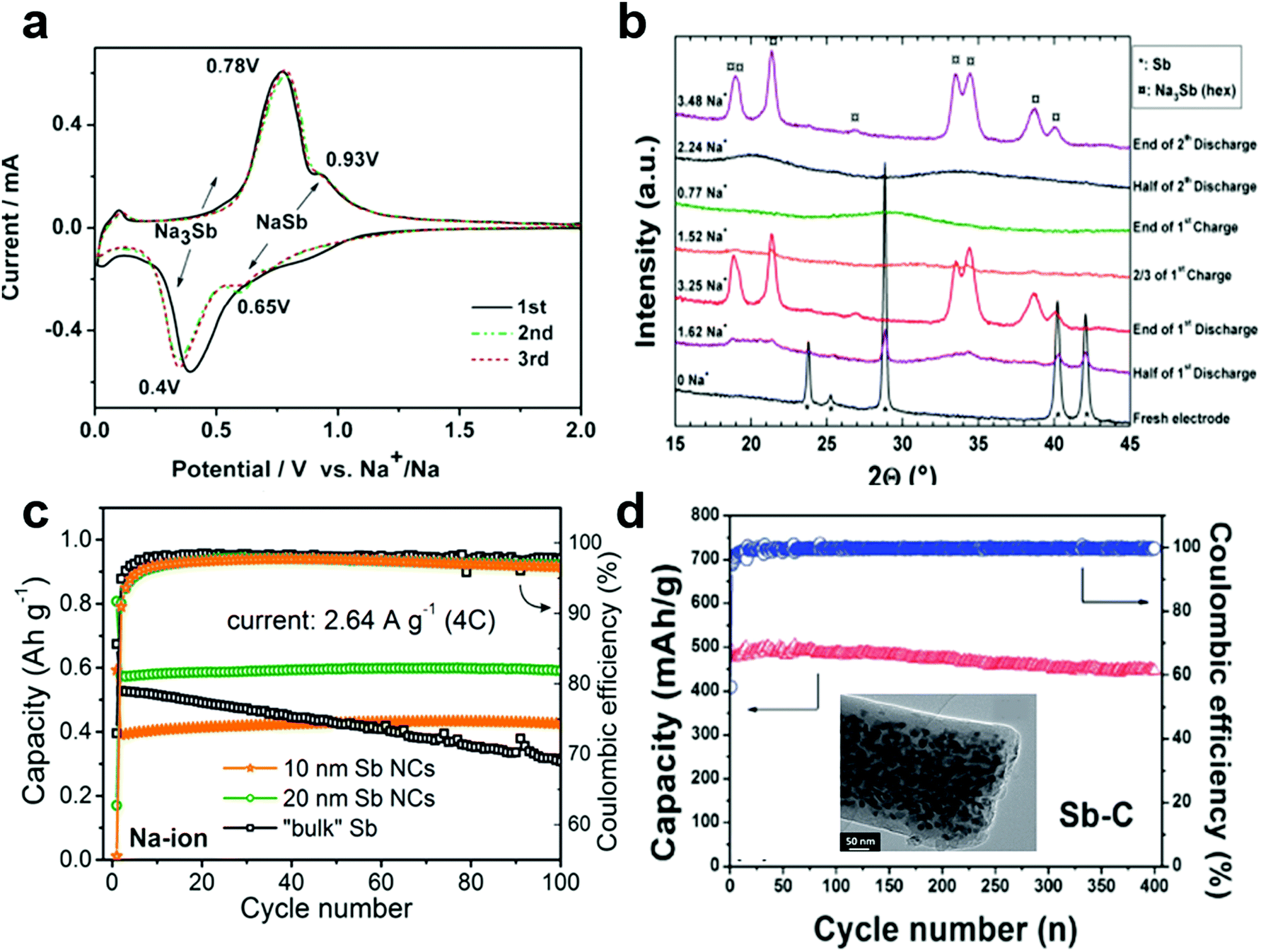
PDF) Nanometer-size Na cluster formation in micropore of hard carbon as origin of higher-capacity Na-ion battery

The relationship between the size of the ions in the ionic liquid and... | Download Scientific Diagram
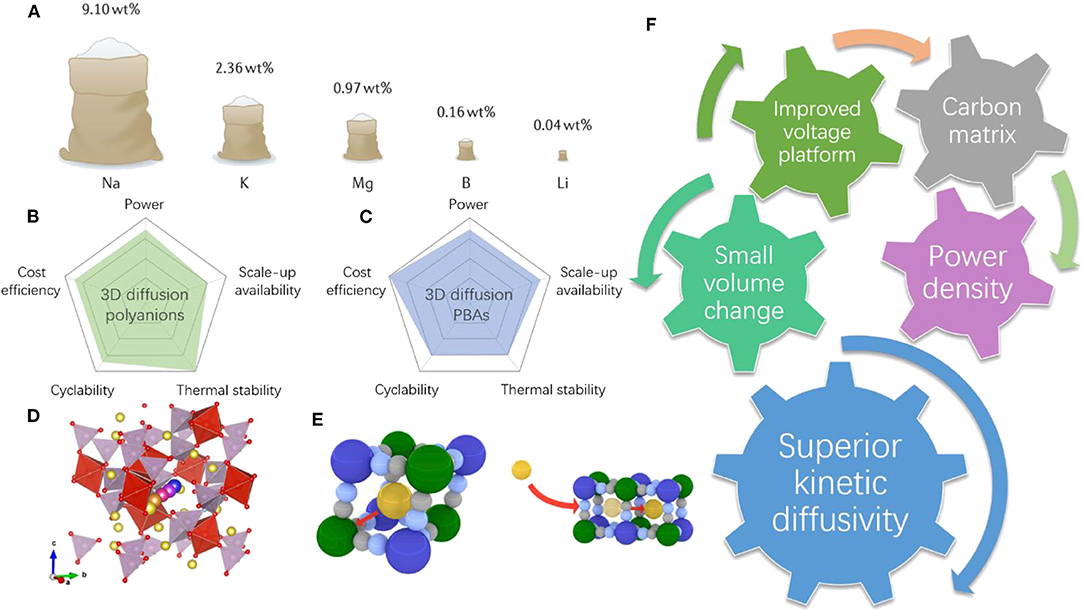
Frontiers | Building High Power Density of Sodium-Ion Batteries: Importance of Multidimensional Diffusion Pathways in Cathode Materials | Chemistry

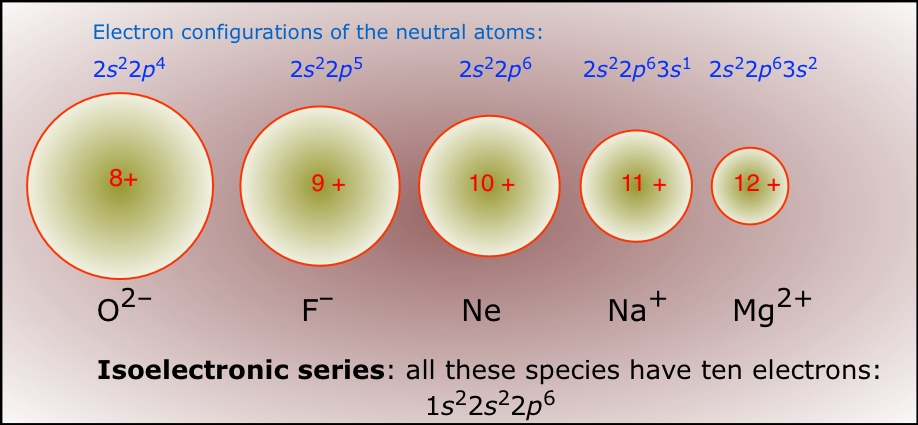
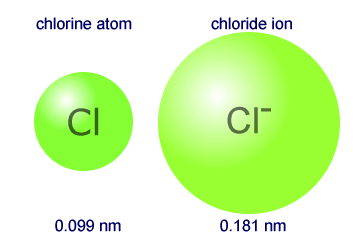
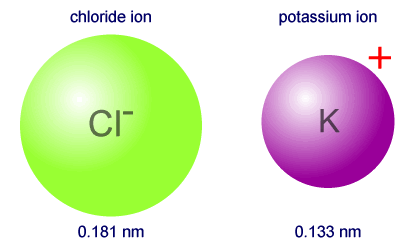
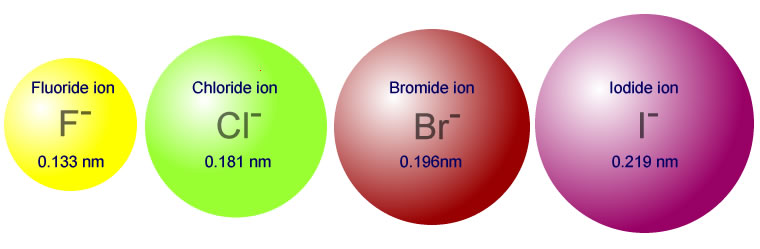
6.3 Periodic Trends Sodium chloride (table salt) produced the geometric pattern in the photograph. Such a pattern can be used to calculate the position. - ppt video online download
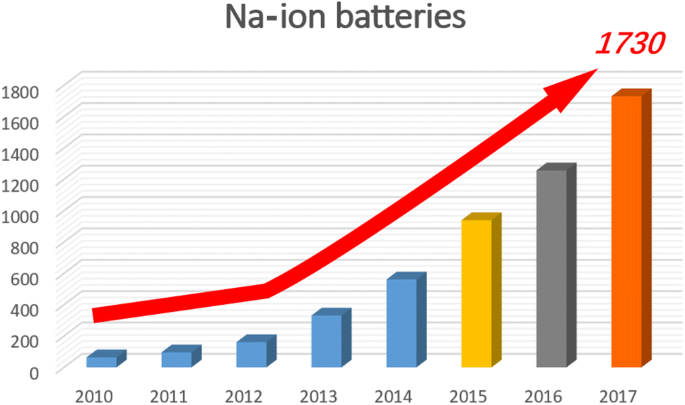
Electrode Materials for Sodium-Ion Batteries: Considerations on Crystal Structures and Sodium Storage Mechanisms | SpringerLink
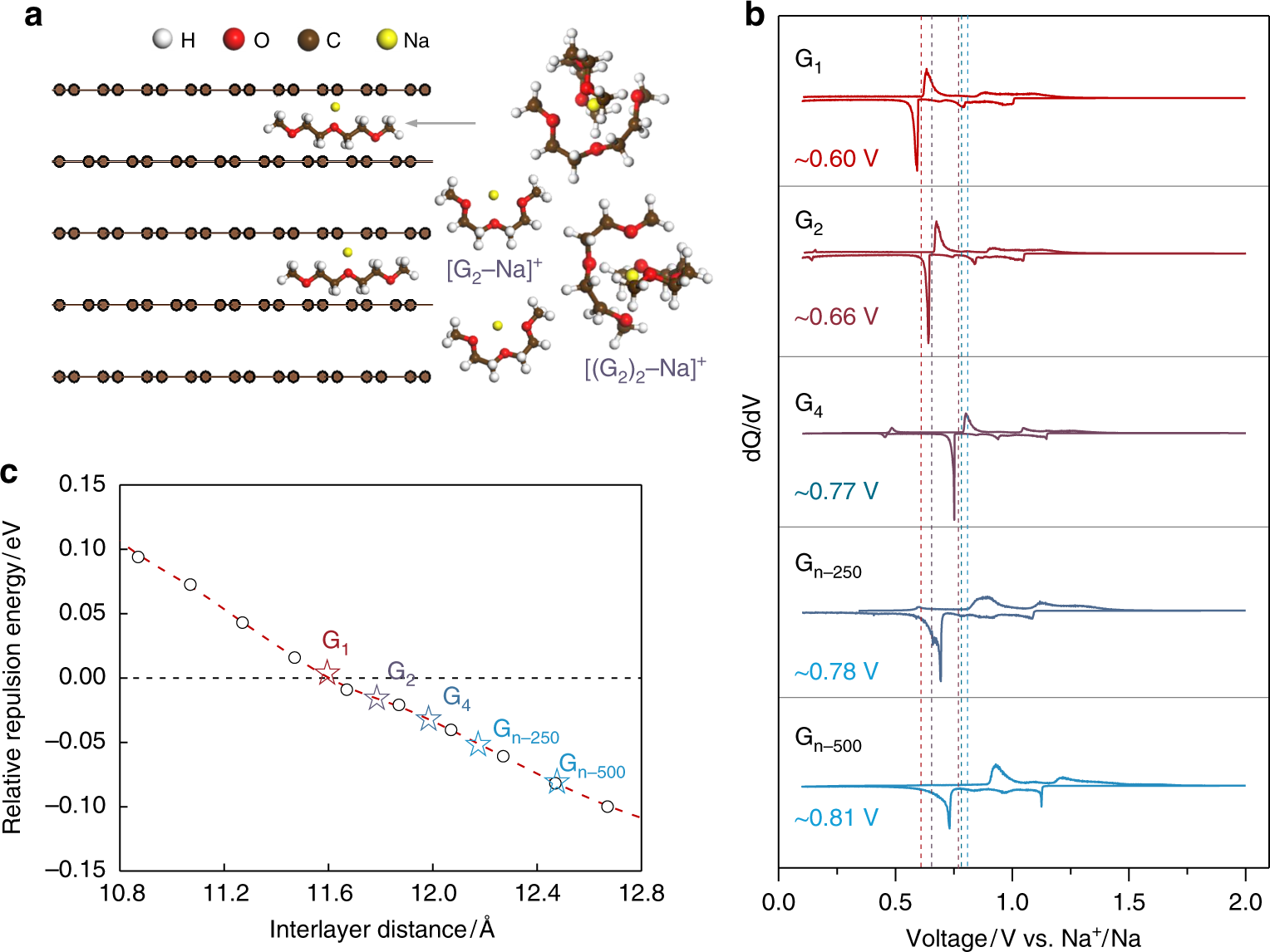
Tailoring sodium intercalation in graphite for high energy and power sodium ion batteries | Nature Communications
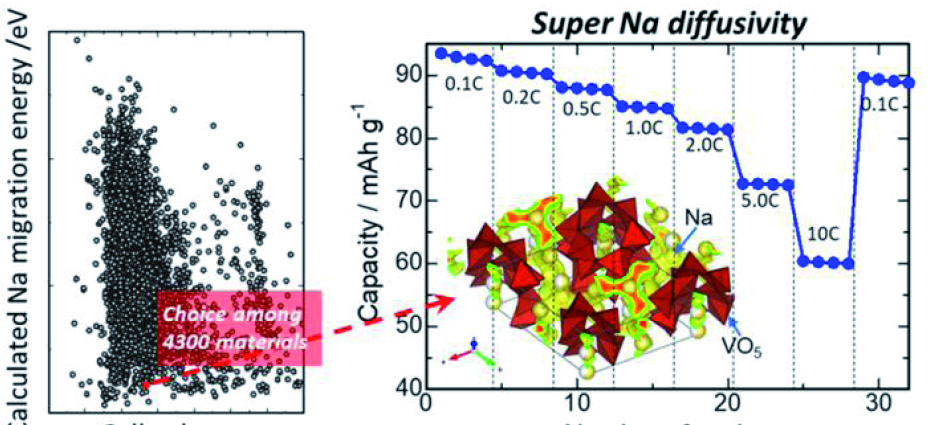
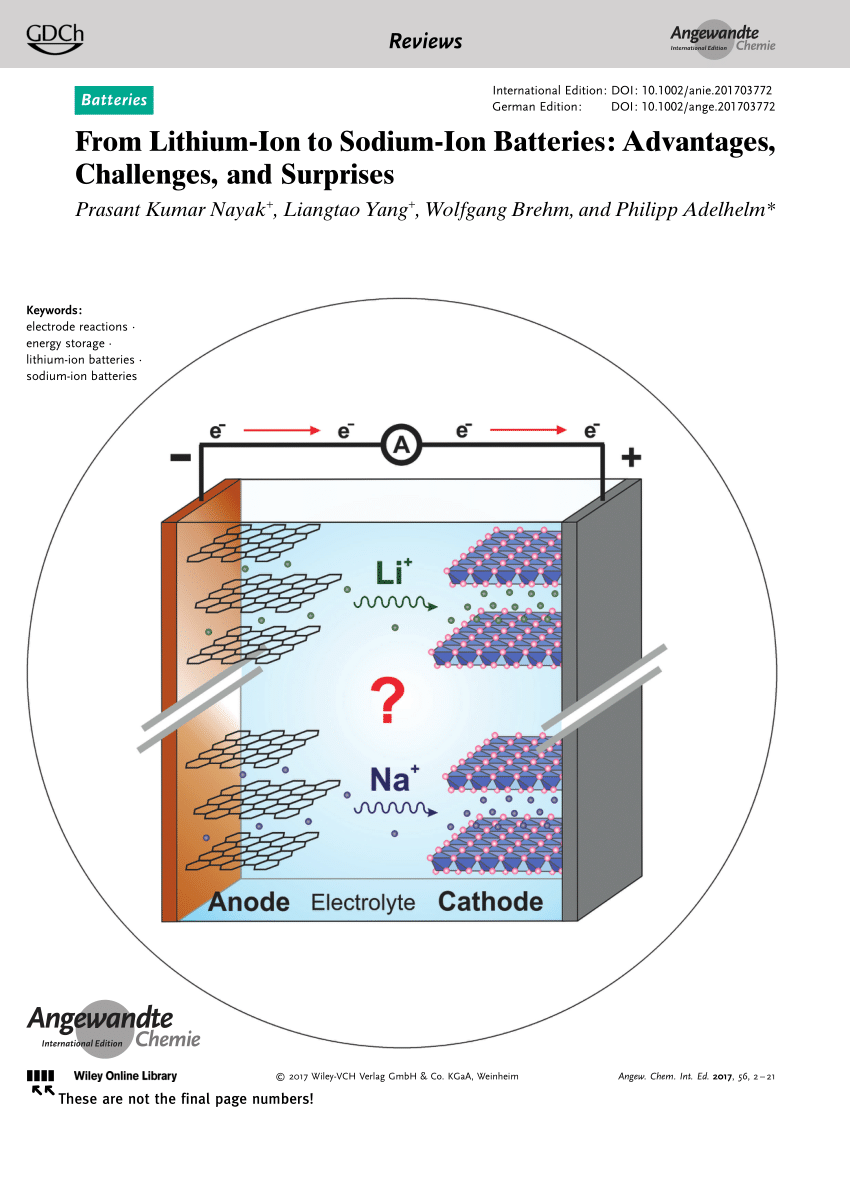
Sodium is the new lithium: Researchers find a way to boost sodium-ion battery performance - eMove360°
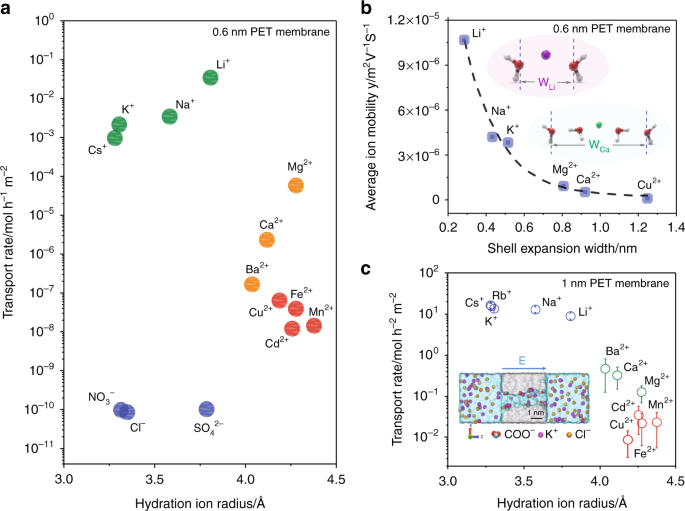
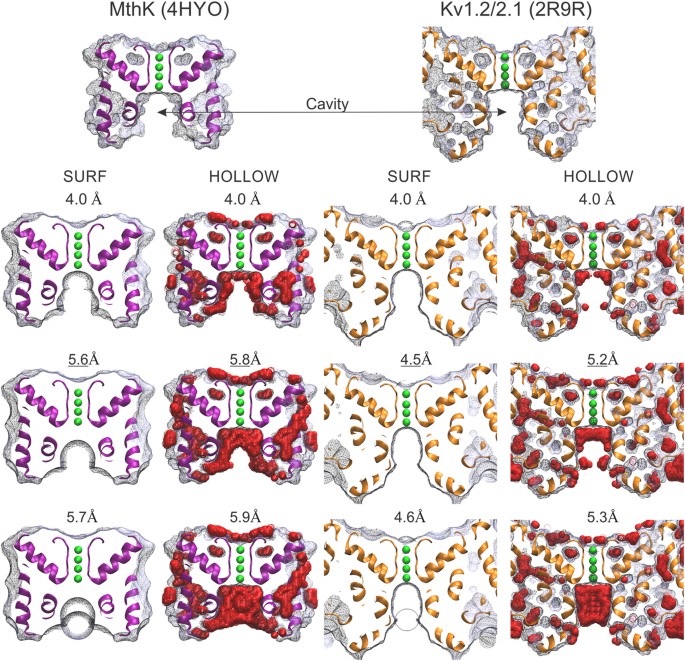
Design principles of ion selective nanostructured membranes for the extraction of lithium ions | Nature Communications